
Have you ever noticed that students get stuck when it comes to the Periodic Table? And you know that there’s no way around it, they MUST get it if they’ve to have any hope of succeeding in science.
And I get it, at first glance, it looks confusing, symbols that don’t sound like the element (although read on to use this to your advantage), numbers that are sometimes whole, sometimes not, and, it turns out that the position of the element is important. Who’d have thunk it!
Like many of us, I’ll show them the Periodic Table song on Youtube (they know it, a few will actually try to remember it). It’s a nice way to get the class started on the right foot.
Next, I would get them to read (or usually I’ll tell the story) of Menedeleev. I’ll always tell them the story of his 1000 mile trek to university with his mother (here’s a sub plan with this). With the background out of the way, it’s time to get down to business.
I use some sneaky tricks to help me win them over.
The first is to use the fact that the element’s symbol and name can, at first glance, look unrelated. It’s a common source of frustration, but you can use it to your advantage.
So, I give them examples. I start with lead (Pb). And I explain that a plumber is called a plumber because they used to work with lead pipes all the time. And I ask them if they’ve heard of a plumb line in construction. And then I ask them, can they guess why lead has the symbol Pb. Plumbum is the latin for lead. Pb is the lead’s nickname.
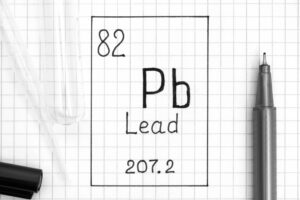
Gold is another one you could use; aurum, shining dawn (not terribly meaningful but a lovely name!). Mercury is named after a Roman god, Plutonium is named after the planet Pluto (it’s always a planet to me). You could get them to guess a lot of them (especially the first 20). The Royal Society of Chemistry has a wealth of information on this. But even using these examples will show them that there’s a reason for the names, it’s not just to make their life harder!
2. Connect the elements to real life:
Helium – they all know!
Copper – used to conduct electricity,
Carbon – all life is based on carbon (and I can’t resist the opportunity to talk about diamonds and the “lead” in pencils).
Lithium – mobile phones, batteries
3. Get them to look at the PT and see if they can see any patterns. They should notice that the elements increase in atomic number. They might be able to see metals on one side of the PT. They may even notice that Group 8 are all gases.
4. Get them to color in the table. This is where you can introduce groups e.g. alkali metals, halogens. Ask them what they have in common (same position in the PT, you can mention that they’ve similar properties). This is a great place to show them the reaction of alkali metals with water and ask them what they notice (more violent reaction as they go down the group).
That’s enough for an intro to the PT. At this stage they know about the history of the PT, they know that elements have nicknames (symbols) and they will have seen that elements are arranged in order of atomic mass (and they’ll also have been introduced to groups).
I hope this has been helpful. Send me a quick email and let me know!
